H2O - Water:
First draw the Lewis dot structure:
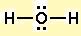
Electron geometry: tetrahedral. Hybridization: sp3
Then draw the 3D molecular structure using VSEPR rules:
Decision:
The molecular geometry of H2O is bent (approx 108 degrees) with asymmetric charge distribution about the central oxygen atom.
Therefore this molecule is polar.
In fact water is one of the most polar molecules there are, and a good illustration of why using VSEPR rules along with electronegativity is important!
Water on Wikipedia.
Back to Molecular Geometries & Polarity Tutorial: Molecular Geometry & Polarity Tutorial.
For homework help in math, chemistry, and physics: www.tutor-homework.com.
