AlCl3 :
First draw the Lewis dot structure:
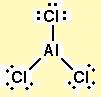
Electron geometry: Trigonal planar. Hybridization: sp2
Next draw the 3D molecular geometry using VSEPR rules:
Decision:
The molecular geometry of AlCl3 is trigonal planar with symmetric electron region distribution.
Therefore this molecule is nonpolar.
Aluminum trichloride at Wikipedia. It should be noted that aluminum trichloride in the solid state is a bit more complicated than the Lewis struckture above. See the Wikipedia article for more info.
Back to Molecular Geometries & Polarity Tutorial: Molecular Geometry & Polarity Tutorial.
For homework help in math, chemistry, and physics: www.tutor-homework.com.
